UV Degradation Mechanisms
As mentioned on our other pages, photons within the UV spectrum possess relatively large amounts of energy due to their short wavelength. When these rays meet a material, this energy can be transfer to the molecules or atoms within the structure, having a range of possible effects.
Polymers such as plastics & rubbers, consist of long molecular chains. These can vary in length, complexity and oientation, and these factors all contribute to the overall material behaviour. Specific polymers possess specific chemical groups and bonds in their structure. Hence, Nylons are characterised by the amide linkage ( -CONH-). These different chemical groups absorb differing amounts of energy. Unfortunately most of these chemical groups have big absorbances within the UV spectrum. The diagram below demonstrates some of the typical chemical groups and their absorption energies.
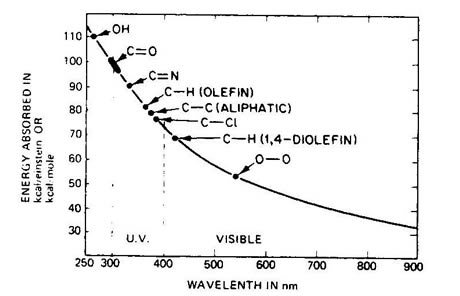
Hence the addition of energy via UV photons can produce significant absoptions of energy by the material and if the energy is sufficient can lead to breaking of the molecular bonds, effectively cutting the molecular chains. These leads to a reduction in molecular weight and subsequent loss in elastic behaviour, cracking and general reduction in the mechanical properties. Gross loss in molecular weight can lead to sticky residues or weeping of chemicals from the surface.
Chalking is another possible degradation mechanism. Chalking appears as a fine powdery residue on the surface of the sample. Typically it is white but can also be other colours depending on the filler. This is caused by the top layer of organic molecules is eroding away, exposing inorganic filler particles or pigment particles leaving them as a dusty or chalky layer of deposit.
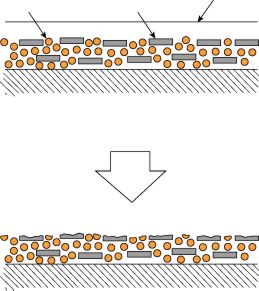
The exact degradation mechanism will vary from polymer to polymer. Some polymers are susceptible to depolymerisation resulting in a systematic reduction in molecular weight which can eventually lead to sticky residues. Other materials may be more random in their degradation and may result in fine cracking of the surface, loss of gloss, stress cracking, or hazing resulting in loss of transparency.
Ceramics are generally less susceptible to UV based damage, although colour changes are not uncommon in the pigments and dyes used on their surfaces. Crazing may be observed in both ceramics and polymers, although it is generally of much greater significance in cermaics due to their lower toughness.
Metals are generally indifferent to UV exposure, although many metallic products will have polymeric coatings applied to them. These coatings or laquers can be severely affected by UV exposure.
